You Develop the Tlc Plate Again and Are Able to Visualize 3 Spots as Shown Below
Thin Layer Chromatography (TLC)
TLC is a unproblematic, quick, and inexpensive procedure that gives the chemist a quick answer equally to how many components are in a mixture. TLC is also used to support the identity of a chemical compound in a mixture when the Rf of a compound is compared with the Rf of a known compound (preferably both run on the aforementioned TLC plate).
A TLC plate is a canvass of glass, metallic, or plastic which is coated with a sparse layer of a solid adsorbent (usually silica or alumina). A small-scale amount of the mixture to be analyzed is spotted near the bottom of this plate. The TLC plate is then placed in a shallow pool of a solvent in a developing bedchamber so that but the very bottom of the plate is in the liquid. This liquid, or the eluent, is the mobile stage, and it slowly rises up the TLC plate by capillary action.
As the solvent moves past the spot that was applied, an equilibrium is established for each component of the mixture between the molecules of that component which are adsorbed on the solid and the molecules which are in solution. In principle, the components will differ in solubility and in the strength of their adsorption to the adsorbent and some components will exist carried farther up the plate than others. When the solvent has reached the top of the plate, the plate is removed from the developing bedchamber, stale, and the separated components of the mixture are visualized. If the compounds are colored, visualization is straightforward. Usually the compounds are not colored, then a UV lamp is used to visualize the plates. (The plate itself contains a fluorescent dye which glows everywhere except where an organic compound is on the plate.)
How To Run a TLC Plate
 | Pace i: Prepare the developing containerThe developing container for TLC tin can be a specially designed chamber, a jar with a chapeau, or a beaker with a sentry glass on the top (the latter is used in the undergrad labs at CU). Pour solvent into the sleeping accommodation to a depth of simply less than 0.5 cm. To aid in the saturation of the TLC bedroom with solvent vapors, you can line part of the inside of the beaker with filter paper. Embrace the beaker with a watch glass, swirl information technology gently, and allow information technology to stand while y'all prepare your TLC plate. |
 | Step 2: Ready the TLC plateTLC plates used in the organic chem teaching labs are purchased as v cm x xx cm sheets. Each big sheet is cut horizontally into plates which are five cm alpine past diverse widths; the more samples you plan to run on a plate, the wider it needs to be. Shown in the photo to the left is a box of TLC plates, a large un-cut TLC sheet, and a minor TLC plate which has been cut to a convenient size. Handle the plates carefully so that you practise not disturb the coating of adsorbent or get them muddied. |
 | Measure 0.5 cm from the bottom of the plate. Using a pencil, describe a line across the plate at the 0.5 cm marker. This is the origin: the line on which you will spot the plate. Take care non to press so difficult with the pencil that you disturb the adsorbent. Under the line, mark lightly the name of the samples you will spot on the plate, or marker numbers for time points. Go out plenty infinite between the samples so that they do non run together; about 4 samples on a 5 cm broad plate is advised. |
 | Step three: Spot the TLC plateIf the sample is not already in solution, dissolve about 1 mg in ane mL of a volatile solvent such equally hexanes, ethyl acetate, or methylene chloride. As a dominion of thumb, a concentration of 1% ordinarily works well for TLC analysis. If the sample is too concentrated, it will run every bit a smear or streak (see troubleshooting section below); if it is not concentrated enough, y'all will run into nothing on the plate. Sometimes you will need to use trial and error to get well-sized, easy to read spots. |
 | Obtain a a microcapillary. In the organic instruction labs, nosotros use 10µL microcaps - they are easier to handle than the smaller ones used in research labs. Dip the microcap into the solution and then gently touch the end of it onto the proper location on the TLC plate. Don't allow the spot to go too large - if necessary, you can touch it to the plate, lift it off and accident on the spot. If y'all echo these steps, the moisture area on the plate volition stay pocket-sized. |
 | This case plate has been spotted with three different quantities of the same solution and is fix to develop. If you are unsure of how much sample to spot, you lot tin always spot multiple quantities and see which looks all-time. |
 | Footstep 4: Develop the plateIdentify the prepared TLC plate in the developing chalice, cover the beaker with the watch glass, and leave it undisturbed on your bench top. The solvent will rising up the TLC plate by capillary action. Make sure the solvent does not cover the spot. |
 | Permit the plate to develop until the solvent is about one-half a centimeter below the top of the plate. Remove the plate from the beaker and immediately marking the solvent front with a pencil. Allow the plate to dry. |
 | Step 5: Visualize the spotsIf there are whatever colored spots, circle them lightly with a pencil. Near samples are not colored and demand to be visualized with a UV lamp. Hold a UV lamp over the plate and circle any spots y'all see. Beware! UV light is damaging both to your eyes and to your skin! Make sure you are wearing your goggles and practice not expect directly into the lamp. Protect your skin past wearing gloves. |
 | If the TLC plate runs samples which are besides concentrated, the spots will be streaked and/or run together. If this happens, y'all will have to beginning over with a more than dilute sample to spot and run on a TLC plate. |
 | Here's what overloaded plates look similar compared to well-spotted plates. The plate on the left has a large yellow smear; this smear contains the aforementioned two compounds which are nicely resolved on the plate adjacent to it. |
TLC Solvents Choice
When yous need to make up one's mind the all-time solvent or mixture of solvents (a "solvent system") to develop a TLC plate or chromatography column loaded with an unknown mixture, vary the polarity of the solvent in several trial runs: a process of trial and mistake. Carefully observe and tape the results of the chromatography in each solvent system. You will detect that as you increase the polarity of the solvent system, all the components of the mixture move faster (and vice versa with lowering the polarity). The platonic solvent system is simply the system that gives the best separation.
TLC elution patterns usually conduct over to column chromatography elution patterns. Since TLC is a much faster procedure than column chromatography, TLC is oftentimes used to determine the best solvent organisation for column chromatography. For instance, in determining the solvent system for a flash chromatography process, the platonic arrangement is the one that moves the desired component of the mixture to a TLC Rf of 0.25-0.35 and volition carve up this component from its nearest neighbour by divergence in TLC Rf values of at least 0.xx. Therefore a mixture is analyzed by TLC to determine the platonic solvent(due south) for a flash chromatography procedure.
Beginners often do not know where to start: What solvents should they pull off the shelf to utilize to elute a TLC plate? Because of toxicity, cost, and flammability concerns, the mutual solvents are hexanes (or petroleum ethers/ligroin) and ethyl acetate (an ester). Diethyl ether tin can be used, just it is very combustible and volatile. Alcohols (methanol, ethanol) can be used. Acetic acid (a carboxylic acrid) can be used, usually as a small percentage component of the system, since it is corrosive, not-volatile, very polar, and has irritating vapors. Acetone (a ketone) tin can be used. Methylene chloride or and chloroform (halogenated hydrocarbons) are good solvents, but are toxic and should be avoided whenever possible. If two solvents are equal in functioning and toxicity, the more volatile solvent is preferred in chromatography because it will be easier to remove from the desired chemical compound later on isolation from a column chromatography procedure.
Inquire the lab teacher what solvents are available and advisable. Then, mix a non-polar solvent (hexanes, a mixture of 6-carbon alkanes) with a polar solvent (ethyl acetate or acetone) in varying percent combinations to make solvent systems of greater and lesser polarity. The charts below should help you in your solvent choice. You can also download this pdf nautical chart of elution order. 

Interactions Between the Compound and the Adsorbent
The strength with which an organic compound binds to an adsorbent depends on the strength of the post-obit types of interactions: ion-dipole, dipole-dipole, hydrogen bonding, dipole induced dipole, and van der Waals forces. With silica gel, the dominant interactive forces between the adsorbent and the materials to be separated are of the dipole-dipole type. Highly polar molecules interact fairly strongly with the polar SiOH groups at the surface of these adsorbents, and will tend to stick or adsorb onto the fine particles of the adsorbent while weakly polar molecules are held less tightly. Weakly polar molecules generally tend to move through the adsorbent more apace than the polar species. Roughly, the compounds follow the elution club given in a higher place.
The Rf value
The retention factor, or Rf, is defined as the distance traveled by the compound divided by the altitude traveled by the solvent.

For example, if a compound travels two.1 cm and the solvent front travels 2.8 cm, the Rf is 0.75:
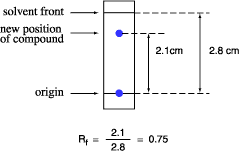
The Rf for a compound is a constant from 1 experiment to the next merely if the chromatography weather below are as well abiding:
- solvent system
- adsorbent
- thickness of the adsorbent
- amount of cloth spotted
- temperature
Since these factors are hard to go along constant from experiment to experiment, relative Rf values are generally considered. "Relative Rf" means that the values are reported relative to a standard, or it means that you compare the Rf values of compounds run on the aforementioned plate at the same time.
The larger an Rf of a compound, the larger the distance it travels on the TLC plate. When comparison two different compounds run under identical chromatography conditions, the compound with the larger Rf is less polar because information technology interacts less strongly with the polar adsorbent on the TLC plate. Conversely, if you know the structures of the compounds in a mixture, you can predict that a compound of low polarity will have a larger Rf value than a polar compound run on the same plate.
The Rf tin can provide corroborative evidence as to the identity of a compound. If the identity of a compound is suspected but not yet proven, an authentic sample of the compound, or standard, is spotted and run on a TLC plate side by side (or on meridian of each other) with the compound in question. If two substances have the same Rf value, they are likely (but not necessarily) the same compound. If they take dissimilar Rf values, they are definitely different compounds. Note that this identity check must be performed on a single plate, because it is difficult to indistinguishable all the factors which influence Rf exactly from experiment to experiment.
Troubleshooting TLC
All of the above (including the process folio) might sound like TLC is quite an piece of cake procedure. But what about the starting time time y'all run a TLC, and run into spots everywhere and blurred, streaked spots? As with whatever technique, with practice you get better. Examples of mutual problems encountered in TLC:
- The compound runs every bit a streak rather than a spot: The sample was overloaded. Run the TLC again subsequently diluting your sample. Or, your sample might just contain many components, creating many spots which run together and appear as a streak. Mayhap, the experiment did not go every bit well as expected.
- The sample runs as a smear or a upward crescent: Compounds which possess strongly acidic or basic groups (amines or carboxylic acids) sometimes show up on a TLC plate with this behavior. Add a few drops of ammonium hydroxide (amines) or acetic acrid (carboxylic acids) to the eluting solvent to obtain clearer plates.
- The sample runs as a downwardly crescent: Probable, the adsorbent was disturbed during the spotting, causing the crescent shape.
- The plate solvent front runs crookedly: Either the adsorbent has flaked off the sides of the plate or the sides of the plate are touching the sides of the container (or the paper used to saturate the container) equally the plate develops. Crooked plates make it harder to measure Rf values accurately.
- Many random spots are seen on the plate: Brand sure that yous do not accidentally drop any organic chemical compound on the plate. If get a TLC plate and leave information technology laying on your workbench equally you do the experiment, you lot might drop or splash an organic chemical compound on the plate.
- You meet a blur of blueish spots on the plate as it develops: Possibly you lot used an ink pen instead of a pencil to mark the origin?
- No spots are seen on the plate: You might not have spotted plenty compound, perhaps because the solution of the compound is also dilute. Try concentrating the solution, or spot it several times in ane place, assuasive the solvent to dry out between applications. Some compounds exercise not evidence upwardly under UV light; try another method of visualizing the plate (such as staining or exposing to iodine vapor). Or, perchance you exercise not have any compound because your experiment did non go as well every bit planned. If the solvent level in the developing jar is deeper than the origin (spotting line) of the TLC plate, the solvent will dissolve the compounds into the solvent reservoir instead of allowing them to move up the plate by capillary activeness. Thus, y'all will not see spots afterwards the plate is developed. These photos show how the yellow compound is running into the solvent when lifted from the developing jar.

TLC Technique Quiz
See how well you understand TLC by taking the online TLC Technique Quiz!
Source: https://www.orgchemboulder.com/Technique/Procedures/TLC/TLC.shtml